What therapeutic equivalence actually means
When your pharmacist hands you a generic pill instead of the brand-name one, you might wonder: is this really the same thing? The answer isn’t just yes or no-it’s backed by science, regulation, and years of real-world data. Therapeutic equivalence means two drugs, even if they look different or cost less, will work the same way in your body. Same effect. Same safety. No surprises.
This isn’t marketing. It’s a strict scientific standard set by the U.S. Food and Drug Administration (FDA). For a generic drug to be labeled therapeutically equivalent, it must meet three rules: it has to have the exact same active ingredient, be absorbed into your bloodstream at the same rate and amount as the brand-name version, and produce the same clinical results in patients. That’s it. No wiggle room.
How the FDA checks if drugs are truly equivalent
The FDA doesn’t guess. They test. Every generic drug must pass bioequivalence studies before approval. These aren’t simple comparisons-they’re controlled clinical trials where healthy volunteers take both the brand and generic versions. Blood samples are taken over hours to measure how much of the drug enters the bloodstream and how fast.
The numbers have to match within tight limits: the area under the curve (AUC) and peak concentration (Cmax) of the generic must fall between 80% and 125% of the brand-name drug. That’s not a guess-it’s a statistical requirement backed by decades of data. For most drugs, this margin is safe. But for drugs like warfarin or levothyroxine, where tiny changes can cause big problems, the FDA tightens the rules to 90-110%.
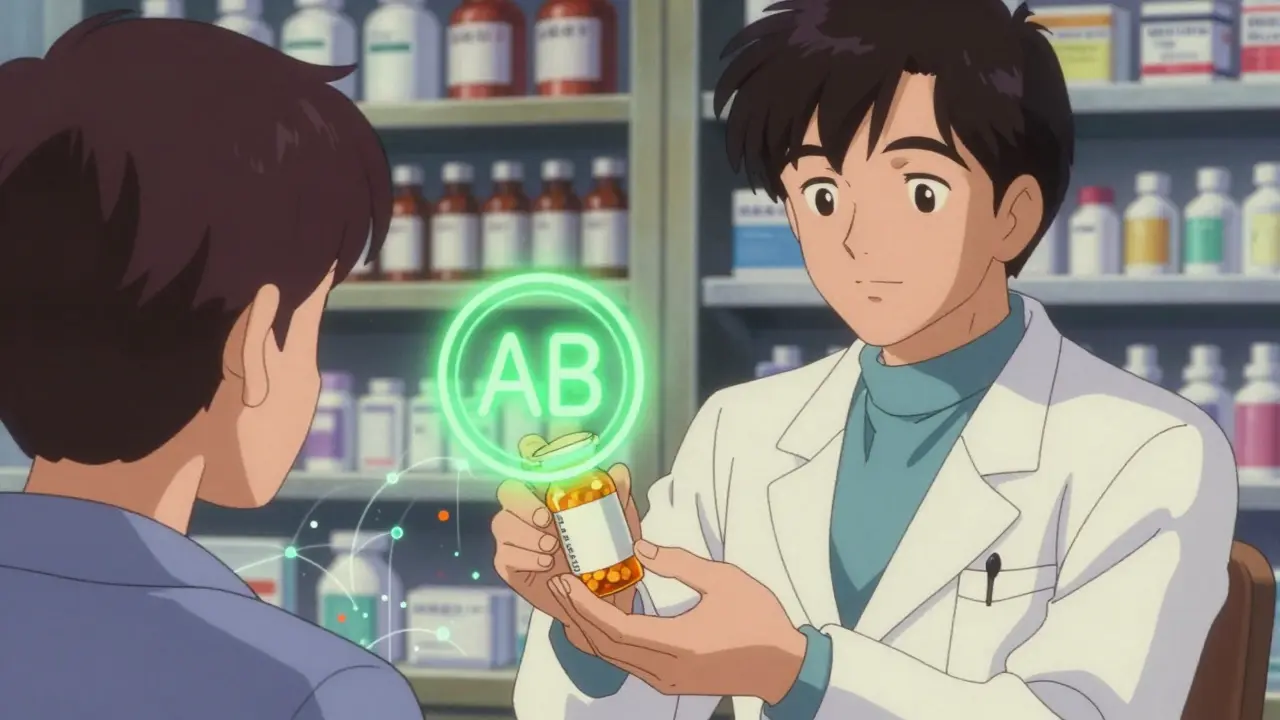
These tests are done in labs with precision equipment, not in clinics. The FDA’s Orange Book, updated every month, lists over 13,000 drug products with their therapeutic equivalence ratings. You’ll see codes like ‘AB’-meaning the generic is approved as interchangeable with the brand. A ‘B’ code? That’s a red flag. It means the generic hasn’t proven equivalence, and shouldn’t be swapped.
Why this matters more than you think
Imagine you’re on a drug that keeps your heart rhythm stable. You’ve been on the same brand for years. Then, your insurance switches you to a generic. If that generic isn’t truly equivalent, your heart could flutter. Or worse. That’s why therapeutic equivalence isn’t just about saving money-it’s about preventing harm.
Studies show that when substitutions happen between drugs that are truly equivalent, adverse events drop. A 2022 UnitedHealthcare survey of 12,500 patients found 87% noticed no difference after switching to an FDA-approved generic. Only 3.2% reported side effects they linked to the change. And when investigators dug into those cases, most weren’t caused by the drug-they were caused by anxiety, confusion, or switching to a non-equivalent product.
The Institute for Safe Medication Practices tracked 127 adverse events tied to generic switches between 2018 and 2022. Only 17 involved products with an ‘A’ rating. The rest? They were either ‘B’ rated or not generics at all-just different drugs from the same class. That’s a key point: therapeutic equivalence isn’t the same as therapeutic interchange. One means same drug, same effect. The other means same category, different molecule. Mixing them up can be dangerous.

What happens when the system breaks down
Most of the time, the system works. But when it doesn’t, the consequences are real. In 2020, a batch of generic levothyroxine had slightly different fillers. Not enough to fail bioequivalence tests-but enough to cause subtle shifts in thyroid hormone levels in sensitive patients. Some reported fatigue, weight gain, or heart palpitations. The FDA didn’t pull the product, but they did tighten the requirements for future submissions.
Complex drugs are the biggest challenge. Inhalers, topical creams, eye drops-these don’t absorb the same way pills do. You can’t just measure blood levels. The FDA admits this. That’s why they’re investing $65 million through 2027 to develop new testing methods. In 2023, they released draft guidance for topical corticosteroids, requiring skin absorption studies instead of just blood tests.
And then there’s the human factor. Patients sometimes think a different color or shape means a different drug. Pharmacists get busy. Prescribers don’t always check the Orange Book. That’s why training matters. The FDA’s free online modules help pharmacists understand the two-letter codes. After training, 85% of participants got the ratings right-up from 52% before.
What you should know as a patient
You don’t need to be a scientist to protect yourself. Here’s what to do:
- Ask your pharmacist: Is this generic approved as therapeutically equivalent? If they hesitate, ask to see the Orange Book code.
- Check your prescription label. If it says “substitutable” or “therapeutically equivalent,” that’s a good sign.
- If you switch and feel different-fatigue, dizziness, mood changes-don’t assume it’s “just in your head.” Call your doctor. Keep a symptom log.
- For narrow therapeutic index drugs (warfarin, lithium, levothyroxine, phenytoin), ask your doctor to write “Dispense as written” on the prescription. That blocks automatic substitution.
- Don’t confuse generics with biosimilars. Biosimilars are for biologic drugs (like Humira). They’re not the same as traditional generics. The FDA has a separate ‘interchangeability’ label for those.
The bigger picture: savings without sacrifice
Therapeutic equivalence isn’t just about safety-it’s about access. In 2022, 90.7% of all prescriptions filled in the U.S. were generics. That’s over 4 billion prescriptions. And yet, generics only made up 58% of total drug spending. Why? Because they cost 80-90% less. That’s $158 billion saved every year.
That money keeps people on their meds. It keeps hospitals from cutting staff. It keeps insurance premiums lower. But none of that matters if the drugs don’t work. Therapeutic equivalence is the bridge between affordability and safety. It’s what lets a single mother in Durban refill her blood pressure pill for $5 instead of $150-and still know it’s doing exactly what it should.
It’s not perfect. There are gaps. Complex drugs still challenge the system. Patient fears are real. But the data is clear: when drugs are truly equivalent, outcomes are the same. And that’s not just policy-it’s proof.
What’s next for therapeutic equivalence
The FDA is teaming up with MIT to build AI models that predict which drug formulations might cause hidden issues. They’re testing machine learning on thousands of past bioequivalence studies to spot patterns humans miss. This could catch problems before they reach shelves.
Meanwhile, states are updating laws. Forty-nine allow pharmacists to swap generics without asking the doctor. Eleven require the prescriber to block substitution. That’s a patchwork. But the core standard-the FDA’s therapeutic equivalence code-remains the gold standard.
As more complex drugs enter the market-biosimilars, inhalers, injectables-the system will adapt. But the goal stays the same: safe, effective, affordable medicine for everyone. Not a promise. A proven system.
Are generic drugs really as safe as brand-name drugs?
Yes-when they’re rated as therapeutically equivalent by the FDA. The FDA requires generics to have the same active ingredient, be absorbed at the same rate and amount, and produce the same clinical results. Over 90% of prescriptions in the U.S. are generics, and studies show no meaningful difference in safety or effectiveness when substitution follows therapeutic equivalence standards.
What does the ‘AB’ code mean on the FDA Orange Book?
The ‘AB’ code means the generic drug has been evaluated and found to be therapeutically equivalent to the brand-name drug. The ‘A’ means there’s no evidence the drug performs differently. The ‘B’ would mean there’s reason to suspect it doesn’t. ‘AB’ is the green light for substitution.
Can I switch between different generic brands of the same drug?
Yes-if both are rated ‘AB’ by the FDA. The system allows multiple generics of the same brand to be interchangeable. But if you’re on a narrow therapeutic index drug like warfarin or levothyroxine, frequent switches-even between approved generics-can sometimes cause small fluctuations. Talk to your doctor about sticking with one manufacturer if you notice changes.
Why do some people say generics don’t work for them?
Most of the time, it’s not the drug. It’s the switch itself. Changing pill size, color, or even the time of day you take it can trigger anxiety or placebo effects. Sometimes, people confuse therapeutic equivalence with therapeutic interchange-switching to a different drug in the same class, which isn’t safe. Rarely, a generic might have a formulation issue, but those are flagged and pulled quickly by the FDA.
What should I do if my insurance forces me to switch to a generic?
Ask your pharmacist to confirm the generic has an ‘AB’ rating in the FDA Orange Book. If you’re on a high-risk medication, ask your doctor to write ‘Dispense as written’ on the prescription. That prevents automatic substitution. You also have the right to pay out-of-pocket for the brand if you’re concerned-insurance can’t force you to take a drug you don’t trust.
Therapeutic equivalence isn’t a buzzword. It’s a lifeline. It’s how millions of people get the medicine they need without risking their health. And as long as the FDA keeps testing, updating, and enforcing these standards, it will keep working.




Kamlesh Chauhan
7 January, 2026 16:23 PMbro why are we even talking about this like its some big deal i got my blood pressure med from walmart for 4 bucks and i aint dead yet lol
Kyle King
8 January, 2026 19:21 PMyou think the FDA is protecting you? nah. they're just keeping the pharma giants happy. remember when they approved that generic Adderall that turned half the country into zombies? they buried the reports. they always do. you think your 'AB' rating means anything when the fillers are made in a factory that also makes rat poison? i've seen the invoices.
Christine Joy Chicano
9 January, 2026 07:26 AMThe FDA's bioequivalence thresholds are actually remarkably conservative. The 80-125% range isn't arbitrary-it's derived from pharmacokinetic modeling that accounts for inter-individual variability in metabolism. What's more impressive is how rarely clinically significant deviations occur. The real issue isn't the science-it's the misinformation that turns a routine pharmacy substitution into a psychological crisis. People panic because the pill looks different, not because it works differently.
Mina Murray
11 January, 2026 00:14 AMLet me break this down for you people who think the system works. The FDA doesn't test on real patients. They test on 24 healthy college kids who get paid to swallow pills. Then they extrapolate to 70-year-olds with three chronic conditions. That's not science. That's a math trick. And don't get me started on the fact that the Orange Book doesn't even include the manufacturing lot numbers. You think your 'AB' rating means your pill came from the same batch as last month? Nope. It could've been made in a different country with different water. And you're supposed to trust this?
Emma Addison Thomas
11 January, 2026 13:28 PMI'm from London, and I've been on generics for over a decade. My GP here prescribes them without question, and I've never had an issue. I think the fear comes from not understanding the process-not from the process itself. Maybe the real problem is that we're taught to distrust anything cheaper. In the UK, we call that 'brand worship'. It's not rational, but it's human.
Rachel Steward
13 January, 2026 03:20 AMYou're all missing the point. This isn't about safety. It's about control. The pharmaceutical industry doesn't want you to know that $300 pills can be replicated for $5. So they weaponize fear. They fund studies that 'prove' generics cause anxiety. They pay influencers to say 'I switched and my migraines got worse'. It's not the drug-it's the narrative. And you're all falling for it. The FDA doesn't need to be perfect. It just needs to be better than the alternatives. And it is.
Alex Danner
13 January, 2026 15:33 PMI'm a pharmacist. I've dispensed thousands of generics. I've seen patients panic over a different color pill. I've had people cry because they thought their meds 'weren't working' after a switch-only to find out they'd been taking it at 11pm instead of 8am. The science is solid. The system works. The human factor? That's the real challenge. Training pharmacists to explain the Orange Book codes reduced confusion by 70% in my county. Knowledge is the antidote to fear.
Jessie Ann Lambrecht
14 January, 2026 19:13 PMIf you're on warfarin or levothyroxine, stick with one brand. Not because generics are dangerous-but because your body gets used to the exact formulation. Tiny differences in fillers can nudge your levels just enough to throw you off. It's not the FDA's fault. It's biology. And if you're stable? Don't fix what ain't broke. But for everyone else? Save your money. The science is on your side.
Vince Nairn
16 January, 2026 14:33 PMso let me get this straight... you're telling me that if i switch from one generic to another generic of the same drug... and i feel weird... its not the drug its my anxiety? like wow. that sounds like the exact same thing they said about women with chronic pain in the 80s. but okay. sure. whatever. my bad for caring about my own body
Anastasia Novak
17 January, 2026 23:46 PMThe FDA is a joke. They approved a generic version of a diabetes drug that had 15% less active ingredient. The manufacturer claimed it was a 'manufacturing variance'. The FDA said 'close enough'. Then they gave the company a $200 million contract to make their next blockbuster. Coincidence? I think not. You're not getting cheaper medicine-you're getting corporate-sponsored risk.
Ayodeji Williams
18 January, 2026 10:09 AMbro the FDA is just another tool of the deep state 😭 they want you dependent on their approved pills 🤡 i got my meds from a guy in Lagos who sends them in a box with a Nigerian stamp 🇳🇬 they work BETTER than the US ones 💪 trust the street not the system
Jonathan Larson
19 January, 2026 06:54 AMTherapeutic equivalence is not merely a regulatory checkbox. It is a moral imperative. The ability to deliver life-sustaining medication at a fraction of the cost-without compromising clinical outcomes-is one of the few triumphs of modern public health policy. To dismiss it as flawed, without evidence, is not skepticism-it is negligence. The data is transparent. The standards are rigorous. And the lives saved by generics are not abstract statistics-they are mothers, fathers, teachers, and neighbors. We owe it to them to understand, not to fear.