When a safety communication is issued - whether it’s about a drug side effect, a faulty medical device, or a new outbreak - your next step isn’t just to read the notice. It’s to monitor your symptoms carefully. This isn’t about panic. It’s about catching problems early, before they become serious. Millions of people have done this during the pandemic, after vaccine alerts, or following recalls of blood pressure meds or insulin pumps. The key is knowing exactly what to look for, how often to check, and when to act.
Understand What the Safety Communication Means
Not all alerts are the same. The FDA, WHO, or CDC might issue a safety communication about a drug linked to liver damage, a pacemaker with a battery defect, or a batch of vaccines with contamination risks. These aren’t warnings to stop using the product immediately - unless they say so. They’re signals to pay closer attention to your body. For example, if you’re taking a medication and the FDA releases a safety notice about rare but serious heart rhythm changes, you don’t need to quit the drug. But you do need to watch for symptoms like dizziness, palpitations, or fainting. If you’re using a home glucose monitor that’s been recalled for inaccurate readings, you don’t throw it out right away - you start comparing its numbers with a backup device or a lab test. The first rule: Don’t ignore it. Don’t overreact. Just observe.Know What Symptoms to Track
Every safety communication lists specific symptoms or adverse events tied to the risk. These aren’t vague. They’re precise. If the alert is about a diabetes drug causing pancreatitis, it will say: “Watch for severe abdominal pain radiating to the back, nausea, vomiting, and fever.” Here’s what to do:- Find the official notice. Go to the FDA’s website, the CDC’s alerts page, or your pharmacy’s recall portal. Don’t rely on social media or news headlines.
- Copy the exact symptoms listed. Write them down. Put them on your fridge or phone wallpaper.
- Learn what’s normal vs. warning. For instance, mild headaches are common. But sudden, sharp head pain with vision changes? That’s not normal.
Choose Your Monitoring Method
There are two main ways to monitor: active and passive. Active monitoring means someone checks in on you. This is common for healthcare workers exposed to infectious diseases or patients on high-risk drugs. Your doctor, clinic, or employer might call or text you daily. In South Africa, some hospitals use SMS-based systems to check on patients after drug recalls. You answer simple questions: “Any fever?” “Any rash?” “Any chest pain?” Passive monitoring is self-directed. You check yourself. This is what most people do after a drug safety alert. You don’t wait for a call. You take responsibility. For passive monitoring, you need a system:- Use a notebook or a free app like Symptomate or CDC’s v-safe (if available).
- Log symptoms daily - even if nothing’s wrong. “No symptoms” is data too.
- Record timing: Did the headache start after taking the pill? Did the swelling appear after 3 days?
Set a Realistic Schedule
How often you check depends on the risk level. - High risk (e.g., new drug with known fatal side effects): Check daily for the first 14 days. Then every other day for 2 weeks. - Medium risk (e.g., device recall with low failure rate): Check every 2-3 days for 30 days. - Low risk (e.g., minor labeling error): Check once a week for 4 weeks. The CDC’s 2022 Field Epidemiology Manual says most people stop monitoring too soon. Symptoms can show up weeks later. Don’t assume safety after a week. Stick to the timeline.
Know When to Call for Help
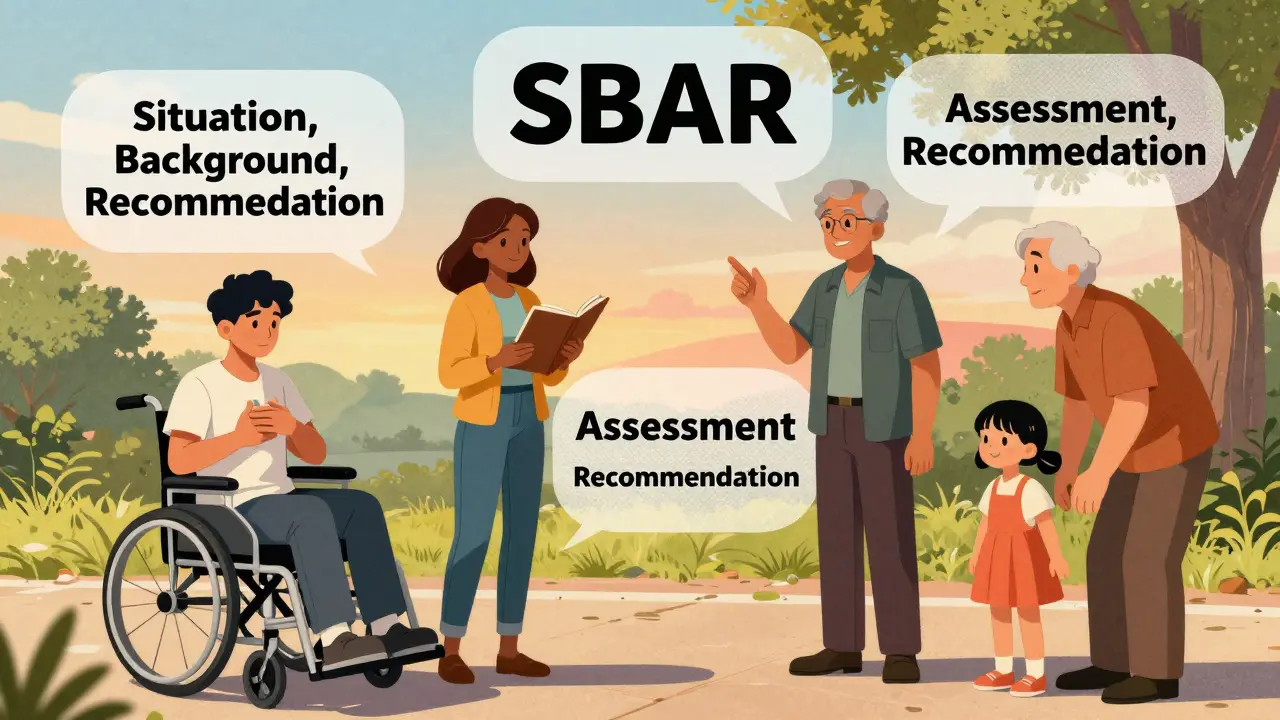
You’re monitoring to catch things early. So when do you act? Use the SBAR method - it’s used in hospitals for a reason:- Situation: “I’m taking Drug X. The FDA issued a safety alert on January 5 about liver injury.”
- Background: “I’ve been on it for 6 weeks. No issues until yesterday.”
- Assessment: “I have yellow eyes, dark urine, and fatigue. My skin looks pale.”
- Recommendation: “I need a liver function test today.”
Document Everything
Write down:- Each symptom (exact wording from the alert)
- Date and time
- Severity (0-10)
- What you did (took meds, rested, drank water)
- What changed (improved, stayed the same, got worse)
Watch Out for Common Mistakes
People mess this up in predictable ways:- Ignoring mild symptoms. “It’s just a little nausea.” But nausea + fatigue + loss of appetite? That’s a pattern.
- Over-monitoring. Checking every hour for a low-risk alert causes anxiety. Stick to the schedule.
- Not tracking “no symptoms.” If you don’t log the absence of symptoms, you can’t prove you’re safe.
- Using unsecured apps. Free apps often sell your data. Use official tools.
- Waiting for someone else to act. If you’re on passive monitoring, you’re the frontline. No one else is watching.
What If You’re Not Tech-Savvy?
You don’t need a smartphone. Paper works. A simple notebook with dates and checkboxes is better than no system at all. Older adults in VA hospitals needed an average of 3.2 help sessions to use digital tools. That’s normal. Ask your pharmacist, nurse, or community health worker for a printed checklist. Many clinics still hand them out. You can also ask a family member to help. One person checks the symptoms, another writes them down. Teamwork beats isolation.How Long Should You Keep Monitoring?
Most people stop after 2-4 weeks. But that’s often too soon. - For drugs: Monitor for at least 60 days. Some side effects appear after 8 weeks. - For devices: Monitor for 90 days. Mechanical issues can be delayed. - For infectious exposures: Follow CDC or local health authority timelines. Some require 14-21 days. If you feel fine after 30 days, don’t assume safety. Slow, delayed reactions happen. Keep checking - even if just once a week.What Happens After Monitoring?
If you notice nothing: Great. You did your part. Keep taking your meds or using your device - unless told otherwise. If you notice something unusual: Contact your doctor. Don’t self-diagnose. Don’t stop treatment without advice. Many symptoms are unrelated to the alert. If you’re told to stop the drug or device: Follow instructions. Then report your experience. The FDA’s MedWatch system lets you file adverse event reports. Your input helps protect others.Final Thought: You’re Part of the System
Safety communications aren’t just for doctors or regulators. They’re for you. Your awareness saves lives - including your own. Monitoring isn’t a chore. It’s a shield. In Durban, where access to healthcare can be uneven, this step becomes even more vital. You can’t always wait for a clinic. But you can watch your body. You can write it down. You can speak up. That’s how public health works - one person, one symptom, one alert at a time.What if I miss a day of symptom tracking?
Missing one day isn’t a failure. Just resume your schedule. Don’t try to backfill - it leads to guesswork. Focus on being consistent going forward. If you’re unsure whether a symptom occurred, note it as “uncertain” and keep monitoring.
Can I trust symptom-tracking apps?
Only use apps backed by health authorities like the CDC or your national health department. Avoid free apps that ask for unnecessary permissions. Check if they’re HIPAA-compliant or follow local data protection laws. In South Africa, look for apps registered with the South African Health Products Regulatory Authority (SAHPRA). Apps without clear privacy policies should be avoided.
Do I need to report my symptoms to anyone?
You’re not required to report unless you’re a healthcare provider or part of a clinical trial. But if you experience a serious side effect, reporting it helps regulators. In the U.S., use MedWatch. In South Africa, report to SAHPRA via their online portal. Your report could prevent others from being harmed.
What if my doctor dismisses my symptoms?
Bring your symptom log. Cite the safety communication by name and date. Say: “The FDA issued this alert on [date], and these are the exact symptoms listed.” If they still dismiss it, ask for a referral to a specialist or request a blood test or imaging. You have the right to a second opinion.
Is symptom monitoring only for drugs?
No. It applies to medical devices (like pacemakers, glucose monitors), vaccines, blood products, even food recalls linked to illness. Any official safety notice - from any health agency - should trigger symptom monitoring. The principles are the same: know the risk, track symptoms, act early.




Dusty Weeks
3 January, 2026 01:40 AMthis is lit 🙌 i’ve been tracking my bp meds after that recall and honestly? i was scared. but writing down "no dizziness, no blurred vision" every day made me feel like i had control. even if nothing happened, i felt like i did my part.
Sally Denham-Vaughan
4 January, 2026 02:15 AMi love how this breaks it down without panic. my mom’s 72 and uses a paper log. she says it’s like her daily journal now. "if i don’t write it, it didn’t happen." she’s right. simple tools > fancy apps that sell your data.
Todd Nickel
5 January, 2026 11:08 AMThe structural clarity of this post is exceptional. It systematically dismantles the cognitive dissonance between public health messaging and individual agency. Most people either ignore safety communications entirely or catastrophize them. This framework-observe, log, act-creates a behavioral nudge that’s both evidence-based and psychologically sustainable. The SBAR protocol, in particular, is underutilized in primary care settings and deserves wider dissemination. Moreover, the emphasis on documenting "no symptoms" as data is a subtle but critical epistemological shift in patient-reported outcomes.
Austin Mac-Anabraba
5 January, 2026 21:14 PMLet me guess-this was written by someone who got paid by Big Pharma to make people feel safe taking dangerous drugs. You tell people to "just observe" but never mention that 80% of these "rare side effects" are actually common and covered up. Why do you think the FDA takes 3 years to update labels? Because they don’t want you to stop taking the pill. You’re being manipulated.
Phoebe McKenzie
6 January, 2026 06:31 AMOMG I CRIED READING THIS. I had a stroke last year after my blood pressure med recall and NO ONE TOLD ME TO WATCH FOR DIZZINESS. I thought it was just stress. I almost died because I trusted the system. Now I have a laminated checklist on my fridge and I check it 3x a day. If you’re not doing this, you’re playing Russian roulette with your life.
gerard najera
7 January, 2026 01:00 AMWatch. Log. Act.
Stephen Gikuma
7 January, 2026 19:18 PMThis is just the government’s way of making you think you’re safe while they quietly replace your meds with something worse. You think they care about your liver? They care about stock prices. Every "safety alert" is a PR move to keep you buying. And don’t get me started on the CDC-they’re just a branch of the WHO, and WHO answers to Bill Gates. You think he wants you healthy? He wants you tracked.
Bobby Collins
8 January, 2026 06:52 AMi used an app for a week and it sent my data to some company in india. now i get ads for liver supplements. i swear i didn’t even search for that. i deleted it. paper only. no tech. no tracking. my phone already knows too much about me.
Layla Anna
8 January, 2026 08:43 AMthis made me feel so seen 😭 i’m a single mom and i didn’t know where to start. i printed the checklist from the FDA site and stuck it on the fridge with magnets. my 8yo helps me check off "no chest pain" every night. we turn it into a game. it’s weirdly bonding. thank you for not making this scary. you made it human.
Heather Josey
8 January, 2026 14:19 PMI appreciate the thoughtful, structured approach outlined here. The emphasis on documentation as a form of self-advocacy is both practical and empowering. Especially for individuals navigating complex healthcare systems, having a clear, consistent record can bridge communication gaps with providers and ensure timely intervention. This is public health done right: accessible, actionable, and grounded in patient dignity.
Donna Peplinskie
8 January, 2026 19:05 PMI just wanted to say... thank you... for writing this... with such care... and clarity... I’ve been monitoring my insulin pump since the recall... and honestly... I felt so alone... until I read this... now I share the checklist with my neighbors... and my book club... because... everyone should know this...
Olukayode Oguntulu
9 January, 2026 03:52 AMAh yes, the neoliberal biopolitical apparatus rebranded as "personal responsibility." You tell people to "monitor symptoms" while defunding public health infrastructure. You make the individual the frontline of risk management while the state outsources surveillance to corporate apps. This isn’t empowerment-it’s disciplinary normalization dressed in wellness aesthetics. The real question isn’t how to track symptoms-it’s why we’ve been forced to do so in the first place.
sharad vyas
9 January, 2026 12:06 PMin india, we don’t have apps for this. we have aunties who ask, "kya dard hua?" if you say yes, they show up with ginger tea and a notebook. sometimes that’s the best monitoring system. culture doesn’t need tech to care.