Tag: bioequivalence
Therapeutic Equivalence: What It Really Means for Patient Safety
Therapeutic equivalence ensures generic drugs work just like brand-name ones, saving money without sacrificing safety. Learn how the FDA verifies this and what it means for your health.
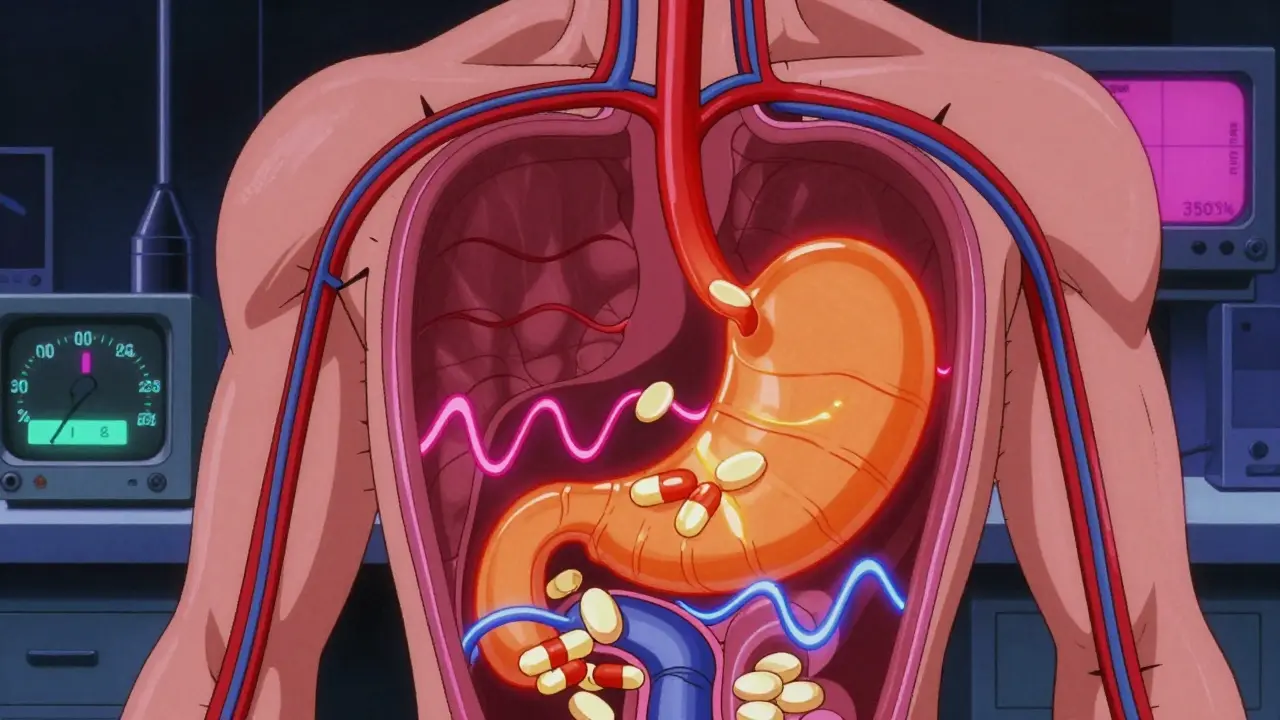
Pharmacokinetic Studies: The Real Standard for Proving Generic Drug Equivalence
Pharmacokinetic studies are the main way regulators prove generic drugs work like brand-name versions. But they're not perfect. For some drugs, blood tests don't tell the whole story-and patient safety depends on knowing when they fall short.
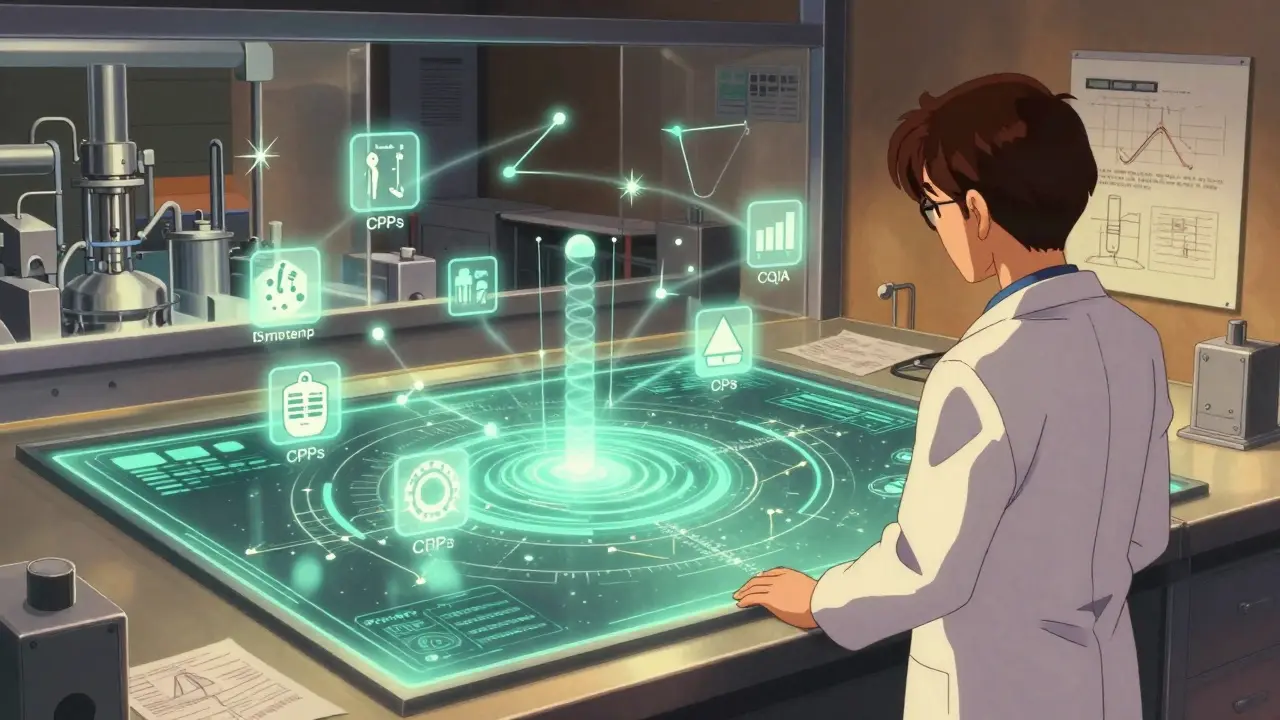
Quality by Design in Generic Drug Development: Modern Science-Based Approaches
Quality by Design (QbD) is now mandatory for generic drug development, transforming how manufacturers prove bioequivalence. Learn how science-based approaches like design space, CQAs, and PAT reduce costs, speed approvals, and improve product consistency.
False Advertising in Generics: Legal Risks and Rules You Can't Ignore
False advertising in generic drugs misleads patients, violates FDA rules, and triggers lawsuits. Learn the legal risks, recent crackdowns, and compliance rules that could save your business or your health.
Complex Generic Drugs: Why Some Products Are Harder to Approve
Complex generic drugs face unique scientific and regulatory hurdles that make approval far harder than for simple generics. From liposomal injections to inhalers, these drugs require advanced testing, unclear guidelines, and millions in investment-delaying affordable access for patients.